Physics[edit]
Bohr model[edit]
Main article: Bohr model
In 1911, Bohr travelled to England. At the time, it was where most of the theoretical work on the structure of atoms and molecules was being done.[21] He met J. J. Thomson of the Cavendish Laboratory and Trinity College, Cambridge. He attended lectures on electromagnetism given by James Jeans and Joseph Larmor, and did some research on cathode rays, but failed to impress Thomson.[22][23] He had more success with younger physicists like the Australian William Lawrence Bragg,[24] and New Zealand's Ernest Rutherford, whose 1911 Rutherford model of the atom had challenged Thomson's 1904 plum pudding model.[25] Bohr received an invitation from Rutherford to conduct post-doctoral work at Victoria University of Manchester,[26] where Bohr met George de Hevesy and Charles Galton Darwin (whom Bohr referred to as "the grandson of the real Darwin").[27]
Bohr returned to Denmark in July 1912 for his wedding, and travelled around England and Scotland on his honeymoon. On his return, he became a privatdocent at the University of Copenhagen, giving lectures on thermodynamics.Martin Knudsen put Bohr's name forward for a docent, which was approved in July 1913, and Bohr then began teaching medical students.[28] His three papers, which later became famous as "the trilogy",[26] were published inPhilosophical Magazine in July, September and November of that year.[29][30][31][32] He adapted Rutherford's nuclear structure to Max Planck's quantum theory and so created his Bohr model of the atom.[30]
Planetary models of atoms were not new, but Bohr's treatment was.[33] Taking the 1912 paper by Darwin on the role of electrons in the interaction of alpha particles with a nucleus as his starting point,[34][35] he advanced the theory of electrons travelling in orbits around the atom's nucleus, with the chemical properties of each element being largely determined by the number of electrons in the outer orbits of its atoms.[36] He introduced the idea that an electron could drop from a higher-energy orbit to a lower one, in the process emitting a quantum of discrete energy. This became a basis for what is now known as the old quantum theory.[37]
In 1885, Johann Balmer had come up with his Balmer series to describe the visible spectral lines of a hydrogen atoms:
where λ is the wavelength of the absorbed or emitted light and RH is the Rydberg constant.[38] Balmer's formula was corroborated by the discovery of additional spectral lines, but for thirty years, no one could explain why it worked. In the first paper of his trilogy, Bohr was able to derive it from his model:
where me is the electron's mass, e is its charge, h is Planck's constant and Z is the atom's atomic number (1 for hydrogen).[39]
The model's first hurdle was the Pickering series, lines which did not fit Balmer's formula. When challenged on this by Alfred Fowler, Bohr replied that they were caused by ionised helium, helium atoms with only one electron. The Bohr model was found to work for such ions.[39] Many older physicists, like Thomson, Rayleigh and Hendrik Lorentz, did not like the trilogy, but the younger generation, including Rutherford, David Hilbert, Albert Einstein,Max Born and Arnold Sommerfeld saw it as a breakthrough.[40][41] The trilogy's acceptance was entirely due to its ability to explain phenomena which stymied other models, and to predict results that were subsequently verified by experiments.[42] Today, the Bohr model of the atom has been superseded, but is still the best known model of the atom, as it often appears in high school physics and chemistry texts.[43]
Bohr did not enjoy teaching medical students. He decided to return to Manchester, where Rutherford had offered him a job as a reader in place of Darwin, whose tenure had expired. Bohr accepted. He took a leave of absence from the University of Copenhagen, which he started by taking a holiday in Tyrol with his brother Harald and aunt Hanna Adler. There, he visited the University of Göttingen and the Ludwig Maximilian University of Munich, where he met Sommerfeld and conducted seminars on the trilogy. The First World War broke out while they were in Tyrol, greatly complicating the trip back to Denmark and Bohr's subsequent voyage with Margrethe to England, where he arrived in October 1914. They stayed until July 1916, by which time he had been appointed to the Chair of Theoretical Physics at the University of Copenhagen, a position created especially for him. His docentship was abolished at the same time, so he still had to teach physics to medical students. New professors were formally introduced to King Christian X, who expressed his delight at meeting such a famous football player.[44]



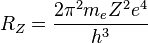
No comments:
Post a Comment