Was it in the water? The jar? The filament........? Or all the above?
Leyden jar
It was invented independently by German cleric Ewald Georg von Kleist on 11 October 1745 and by Dutch scientist Pieter van Musschenbroek of Leiden (Leyden) in 1745–1746.[1] The invention was named after the city.
The Leyden jar was used to conduct many early experiments in electricity, and its discovery was of fundamental importance in the study of electrostatics. The Leyden jar was the first means of storing an electric charge which then could be discharged at the experimenter's will. Leyden jars are still used in education to demonstrate the principles of electrostatics.
Contents
[hide]History[edit]

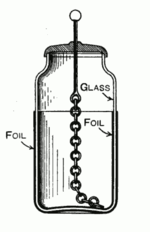
Discovery of the Leyden jar in Musschenbroek's lab. The static electricity produced by the rotating glass sphere electrostatic generator was conducted by the chain through the suspended bar to the water in the glass held by assistant Andreas Cuneus. A large charge accumulated in the water and an opposite charge in Cuneus' hand on the glass. When he touched the wire dipping in the water, he received a powerful shock.
Around 1650, Otto von Guericke built a crude electrostatic generator: a sulphur ball that rotated on a shaft. When Guericke held his hand against the ball and turned the shaft quickly, a static electric charge built up. This experiment inspired the development of several forms of "friction machines", that greatly helped in the study of electricity.
The Leyden jar was effectively discovered independently by two parties: German deacon Ewald Georg von Kleist, who made the first discovery, and Dutch scientists Pieter van Musschenbroek and Andreas Cunaeus, who figured out how it worked only when held in the hand.[3]
Despite its mundane and safe appearance, the Leyden jar is a high voltage device, and electrical energy collected within it from friction may be as high as 35,000 volts. The ball on the tip of the rod is a corona ball to prevent leakage of the energy into the air by point discharge.
Von Kleist[edit]
Ewald Georg von Kleist (aka JG von Kleist) discovered the immense storage capability of the Leyden jar while working under a theory of electricity that saw electricity as a fluid, and hoped a glass jar filled with alcohol would "capture" this fluid.[4] He was the deacon at the cathedral of Camin in Pomerania.In 1744 von Kleist tried to accumulate electricity in a small medicine bottle filled with alcohol with a nail inserted in the cork. He was following up on an experiment developed by Georg Matthias Bose where electricity had been sent though water to set alcoholic spirits alight. He attempted to charge the bottle from a large prime conductor (invented by Bose) suspended above his friction machine.
Kleist was convinced that a substantial electric charge could be collected and held within the glass which he knew would provide an obstacle to the escape of the 'fluid'. He received a significant shock from the device when he accidentally touched the nail through the cork while still cradling the bottle in his other hand. He corresponded with a number of electrical experimenters, but didn't understand the significance of his conducting hand holding the bottle—and both he and his correspondents were loath to hold the device when told that the shock could throw them across the room. It took some time before Kleist's student associates at Leyden worked out that the hand provided an essential element.
Musschenbroek and Cunaeus[edit]
| This section needs additional citations for verification. (July 2016) (Learn how and when to remove this template message) |
Andreas Cuneaus appears to have been the first to receive communications from von Kleist about the storage capacity of the jar. He attempted to duplicate the experiment using a glass of beer, but couldn't make it work. He then worked with the Professor of Physics at Leyden University, and they eventually charged a jar of water with electricity only by holding it in the hand, rather than mounting it on an insulating resin block. Cunaeus and Musschenbroek also received severe shocks, and Musschenbroek communicated the experiment to Abbé Nollet, René Antoine Ferchault de Réaumur, and the wider French scientific community.
Musschenbroek's outlet in France for the sale of his company's 'cabinet' devices was the Abbé Nollet (who ran a similar business). Nollet then gave the electrical storage device the name "Leyden Jar" and promoted it as a special type of flask to his market of wealthy men with scientific curiosity. The "Kleistian jar" was therefore promoted as the Leyden Jar as having been discovered by Pieter van Musschenbroek and his assistant Andreas Cunaeus at the University of Leiden.
Daniel Gralath was the first to connect several jars in parallel to increase the total possible stored charge.[5] The term "battery" was coined by Benjamin Franklin[6] for these combinations, who likened it to a battery of cannon (cannons grouped in a common place).[citation needed] The term was later used for combinations of multiple electrochemical cells, the modern meaning of the term "battery". By the middle of the 19th century, the Leyden jar had become common enough for writers to assume their readers knew of and understood its basic operation.[citation needed]
Around the turn of the century it began to be widely used in spark-gap transmitters and medical electrotherapy equipment. By the early 20th century, improved dielectrics and the need to reduce their size and undesired inductance and resistance for use in the new technology of radio caused the Leyden jar to evolve into the modern compact form of capacitor.
Design[edit]
A typical design consists of a glass jar with conducting tin foil coating the inner and outer surfaces. The foil coatings stop short of the mouth of the jar, to prevent the charge from arcing between the foils. A metal rod electrode projects through the stopper at the mouth of the jar, electrically connected by some means (usually a hanging chain) to the inner foil, to allow it to be charged. The jar is charged by an electrostatic generator, or other source of electric charge, connected to the inner electrode while the outer foil is grounded. The inner and outer surfaces of the jar store equal but opposite charges.[7]The original form of the device was just a glass bottle partially filled with water, with a metal wire passing through a cork closing it. The role of the outer plate was provided by the hand of the experimenter. Soon it was found that it was better to coat the exterior of the jar with metal foil (Watson, 1746), leaving the (accidentally) impure water inside acting as a conductor, connected by a chain or wire to an external terminal, a sphere to avoid losses by corona discharge. Later the water inside was replaced with a second metal foil lining. Early experimenters[who?] found[when?] that the thinner the dielectric, the closer the plates, and the greater the surface, the greater the charge that could be stored at a given voltage.[citation needed]
Further developments in electrostatics revealed that the dielectric material was not essential, but increased the storage capability (capacitance) and prevented arcing between the plates. Two plates separated by a small distance also act as a capacitor, even in a vacuum.
Originally, the amount of capacitance was measured in number of 'jars' of a given size, or through the total coated area, assuming reasonably standard thickness and composition of the glass. A typical Leyden jar of one pint size has a capacitance of about 1 nF.
Storage of the charge[edit]
It was initially believed that the charge was stored in the water in early Leyden jars. In the 1700s American statesman and scientist Benjamin Franklin performed extensive investigations of both water-filled and foil Leyden jars, which led him to conclude that the charge was stored in the glass, not in the water. A popular experiment, due to Franklin, which seems to demonstrate this involves taking a jar apart after it has been charged and showing that little charge can be found on the metal plates, and therefore it must be in the dielectric. The first documented instance of this demonstration is in a 1749 letter by Franklin.[8] Franklin designed a "dissectible" Leyden jar (right), which was widely used in demonstrations. The jar is constructed out of a glass cup nested between two fairly snugly fitting metal cups. When the jar is charged with a high voltage and carefully dismantled, it is discovered that all the parts may be freely handled without discharging the jar. If the pieces are re-assembled, a large spark may still be obtained from it.This demonstration appears to suggest that capacitors store their charge inside their dielectric. This theory was taught throughout the 1800s. However, this phenomenon is a special effect caused by the high voltage on the Leyden jar.[9] In the dissectible Leyden jar, charge is transferred to the surface of the glass cup by corona discharge when the jar is disassembled; this is the source of the residual charge after the jar is reassembled. Handling the cup while disassembled does not provide enough contact to remove all the surface charge. Soda glass is hygroscopic and forms a partially conductive coating on its surface, which holds the charge.[9] Addenbrook (1922) found that in a dissectible jar made of paraffin wax, or glass baked to remove moisture, the charge remained on the metal plates.[10] Zeleny (1944) confirmed these results and observed the corona charge transfer.[11] In capacitors generally, the charge is not stored in the dielectric, but on the inside surfaces of the plates, as can be observed from capacitors that can function with a vacuum between their plates.[12]





No comments:
Post a Comment